For localized information and support, would you like to switch to your country-specific website for {0}?
Roche receives FDA approval for VENTANA FOLR1 (FOLR1-2.1) RxDx Assay as the first IHC-based companion diagnostic to identify ovarian cancer patients eligible for ELAHERE
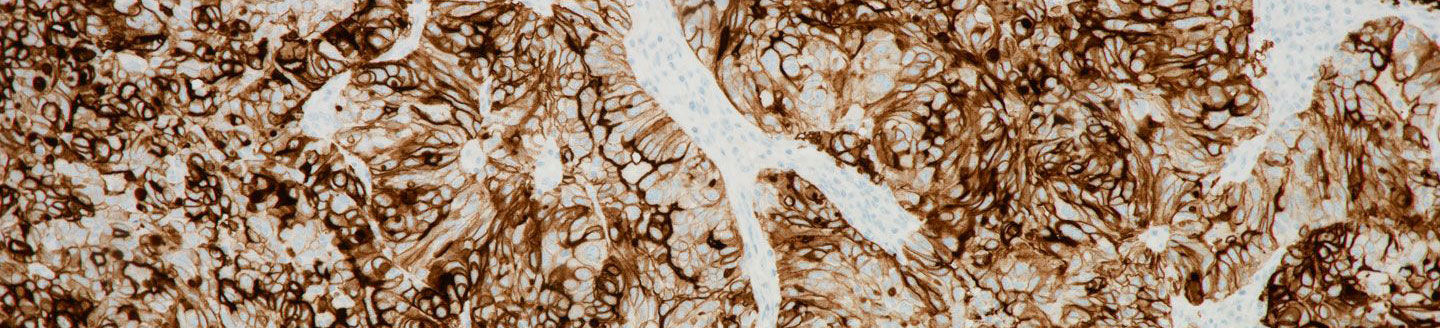
- Developed as a predictive biomarker, the VENTANA FOLR1 (FOLR1-2.1) RxDx Assay detects the folate receptor 1 protein (FOLR1 or FRɑ), which is over-expressed in most ovarian cancers.
- The new test identifies ovarian cancer patients eligible for targeted treatment with ELAHERE.
- The test is the latest addition to Roche’s portfolio of companion diagnosticsdesigned to provide critical insights that enable more informed clinical decisions and improved patient outcomes.
Roche (SIX: RO, ROG; OTCQX: RHHBY) today announced US Food and Drug Administration (FDA) approval of the VENTANA FOLR1 (FOLR1-2.1) RxDx Assay, the first immunohistochemistry (IHC) companion diagnostic test to aid in identifying epithelial ovarian cancer (EOC) patients who are eligible for targeted treatment with ELAHERE™ (mirvetuximab soravtansine-gynx). ELAHERE is a first-in-class antibody-drug conjugate (ADC) therapy developed by ImmunoGen, Inc., approved under FDA’s Accelerated Approval program for the treatment of FRɑ-positive platinum-resistant ovarian cancer.
Folate receptor 1 protein (FOLR1), also known as folate receptor alpha (FRɑ), is expressed at some level in approximately 90 percent of ovarian carcinomas and serves as a predictive biomarker for FOLR1-targeted therapy for EOC patients.1,2 The new test informs clinicians about the likelihood of potential patient benefit from FOLR1 therapy,3,4 advancing Roche’s commitment to personalised healthcare through innovative solutions that help fit the treatment to the individual.
“We’re proud to expand our women’s health and oncology portfolios through the addition of the first companion diagnostic IHC test for ovarian cancer,” said Jill German, Head of Pathology Lab at Roche Diagnostics. “This test will enable clinicians to make more informed treatment decisions for patients with ovarian cancer by quickly determining whether they qualify for ELAHERE therapy, potentially improving their outcomes.”
The approval is based on the results of the SORAYA clinical study.4 In the study, approximately 35% of ovarian cancer patients expressed high levels of FRα (defined as ≥ 75% tumour cells staining with 2+/3+ intensity) and were considered FRα-positive by the VENTANA FOLR1 (FOLR1-2.1) RxDx Assay. Of the FRα-positive patients, about 32 percent demonstrated a partial or complete response to ELAHERE therapy.5
Ovarian cancer is the eighth most common cancer in women worldwide and patients often have advanced disease when diagnosed.6 Despite improvements in primary therapy, mortality rates remain high, and 80 percent of patients with advanced EOC will have their disease recur.7,8 The five-year relative survival rate for all stages of invasive EOC is 49 percent.9
The launch of Roche’s first companion test for ovarian cancer highlights the company’s commitment, as the world’s leading provider of in vitro diagnostics, to continued innovation and evolution of its products in order to advance personalised healthcare and deliver novel, high medical value solutions that improve patients’ lives. For the current list of labs in the US that offer testing, please visit https://usinfo.roche.com/folr1ihc.html.
About the VENTANA FOLR1 (FOLR1-2.1) RxDx Assay
Roche has developed a leading, comprehensive and differentiated cancer immunohistochemical portfolio, with biomarkers that support multiple guidelines for the diagnosis and stratification of cancers. VENTANA FOLR1 (FOLR-2.1) RxDx Assay is a qualitative immunohistochemical assay using mouse monoclonal anti-FOLR1 clone FOLR1-2.1 intended for use in the assessment of folate receptor alpha (FRɑ) in formalin-fixed, paraffin-embedded epithelial ovarian cancer (EOC), including primary peritoneal cancer and primary fallopian tube cancer, tissue specimens by light microscopy. The OptiView DAB IHC Detection Kit is used for staining on a BenchMark ULTRA instrument.
Founded in 1896 in Basel, Switzerland, as one of the first industrial manufacturers of branded medicines, Roche has grown into the world’s largest biotechnology company and the global leader in in-vitro diagnostics. The company pursues scientific excellence to discover and develop medicines and diagnostics for improving and saving the lives of people around the world. We are a pioneer in personalised healthcare and want to further transform how healthcare is delivered to have an even greater impact. To provide the best care for each person we partner with many stakeholders and combine our strengths in Diagnostics and Pharma with data insights from the clinical practice.
In recognising our endeavour to pursue a long-term perspective in all we do, Roche has been named one of the most sustainable companies in the pharmaceuticals industry by the Dow Jones Sustainability Indices for the thirteenth consecutive year. This distinction also reflects our efforts to improve access to healthcare together with local partners in every country we work.
Genentech, in the United States, is a wholly owned member of the Roche Group. Roche is the majority shareholder in Chugai Pharmaceutical, Japan.
All trademarks used or mentioned in this release are protected by law.
References
- Scaranti, M., Cojocaru, E., Banerjee, S. et al. Exploiting the folate receptor α in oncology. Nat Rev Clin Oncol 17, 349–359 (2020). https://doi.org/10.1038/s41571-020-0339-5.
- Hilgenbrink A., Low P. Folate receptor-mediated drug targeting: From Therapeutics to diagnostics. Journal of Pharmaceutical Sciences. 2005;94(10): 2135-2146.
- James, R., Admire, B., Sisseron, T et al. 1125P Analytical assessment of a diagnostic immunohistochemical assay for the detection of folate receptor-ɑ in epithelial ovarian cancers. 2021; Annals of Oncology, Volume 32, S921 - S922.
- Roche. VENTANA FOLR1 (FOLR-2.1) RxDx Assay. US Package Insert. 2022.
- Matulonis UA, et al. Abstract LB4. Presented at Society of Gynecologic Oncology 2022 Annual Meeting on Women's Cancer. March 18-21, 2022.
- American Cancer Society, “About Ovarian Cancer.”Cancer.org, accessed 15 July 2022. URL https://www.cancer.org/cancer/ovarian-cancer/about.html.
- du Bois A, Herrstedt J, Hardy-Bessard AC, et al. Phase III trial of carboplatin plus paclitaxel with or without gemcitabine in first-line treatment of epithelial ovarian cancer. Journal of Clinical Oncology Clin Oncol. 2010;28(27):4162–4169.
- Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I, Bray F (2020). Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. Available from: https://gco.iarc.fr/today, accessed [15 July 2022].
- American Cancer Society, “About Ovarian Cancer.”Cancer.org, accessed 15 July 2022. URL https://www.cancer.org/cancer/ovarian-cancer/about.html.