For localized information and support, would you like to switch to your country-specific website for {0}?
A powerful portfolio to help end a preventable disease
Committed to the quest to eliminate cervical cancer
Almost all cervical cancer cases are linked to infection with high risk human papillomavirus (HPV), an extremely common virus transmitted through sexual contact.1 Although most HPV infections resolve spontaneously and cause no symptoms, persistent infection can cause cervical cancer. With vaccination, advances in screening, and appropriate treatment, cervical cancer is now a preventable disease.
Unfortunately, barriers still exist that prevent some women and individuals with a cervix from ever being screened for cervical cancer. Roche wants to change that. We envision a world where no one dies from cervical cancer, because access to early and accurate screening, diagnosis and treatment is available to everyone, no matter where they live.
We are at the forefront of developing quality screening, triage, and diagnostics solutions for cervical cancer. Roche is part of a global effort to eradicate cervical cancer and is doing its part by increasing awareness, screening accuracy and linkage to care for all women – no matter where they live. Our comprehensive cervical cancer portfolio covers screening, triage, and diagnostic solutions, including self collection.
A preventable global burden
Despite being nearly 100% preventable with proper HPV vaccination, screening, and treatment, cervical cancer remains one of the main causes of death in women.1 In 2022, approximately 660,000 women worldwide were diagnosed with cervical cancer and about 350,000 died from this preventable disease.1 The highest incidence of cervical cancer cases occur in low- and middle-income countries, mainly attributable to lack of access to HPV vaccinations, cervical screening, and treatment services.
If diagnosed early, cervical cancer can be treated and cured. At Roche, we envision a world where no one dies from cervical cancer, because access to early and accurate screening, diagnosis, and treatment is available to everyone, no matter where they live. Roche’s portfolio, now offering options for self-collection, reaches across geographies and cultures, so no one ever has to die from a preventable disease.
A powerful portfolio for cervical cancer diagnosis
HPV DNA detection is recommended by the World Health Organization (WHO) as the primary screening test to help achieve cervical cancer elimination.2 The WHO also recommends all countries prioritize screening for cervical cancer, above all other cancers.3
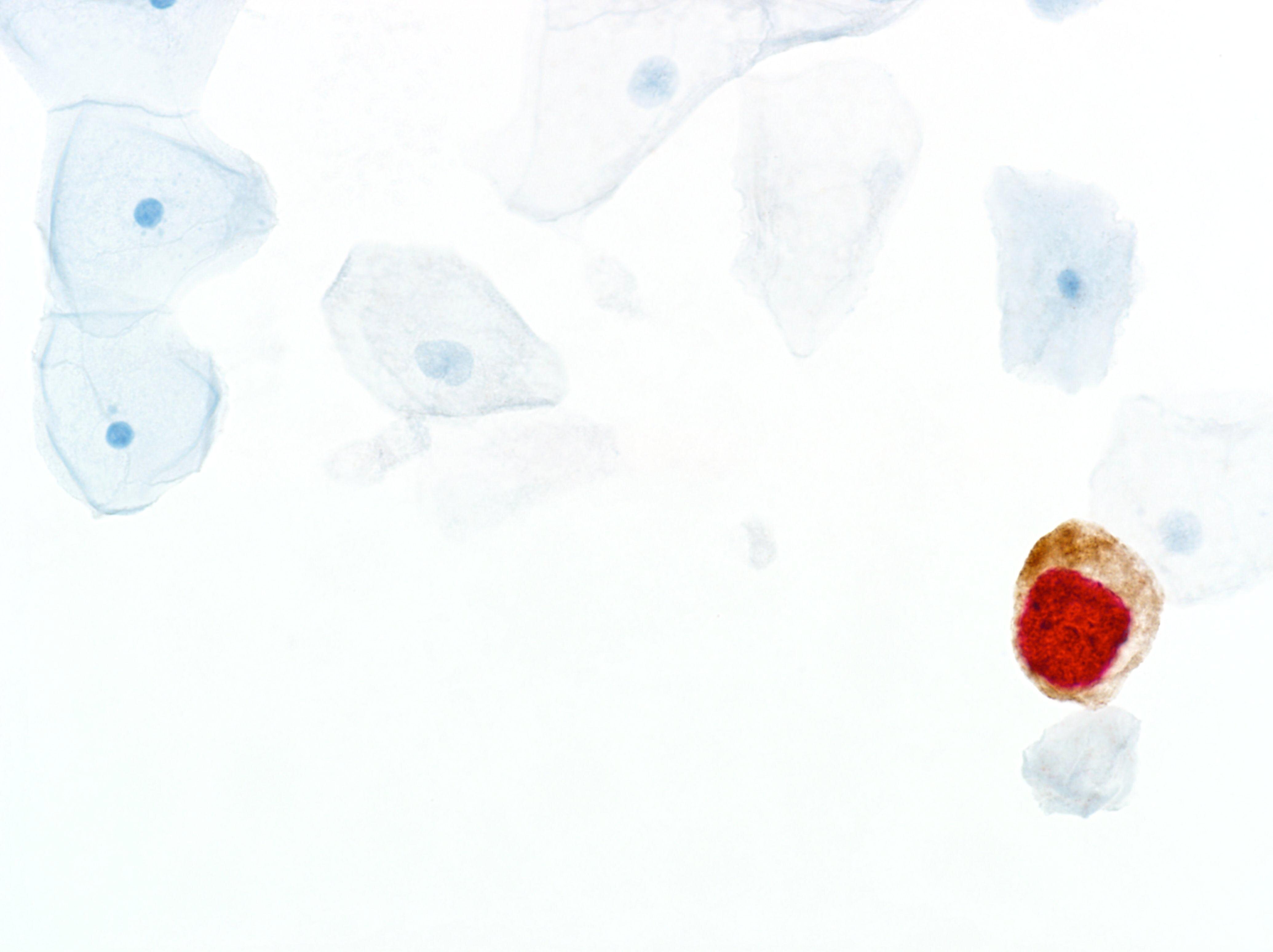
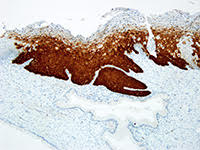
In the quest to eliminate cervical cancer, Roche offers a comprehensive portfolio that goes beyond a single product, offering a strategic path to diagnostic certainty. By leveraging advanced tools such as cobas® HPV for risk assessment, CINtec® PLUS Cytology for immediate triage, and CINtec® Histology for accurate diagnosis, this approach addresses critical points in cervical cancer prevention and management.
With its comprehensive cervical cancer screening portfolio, Roche delivers powerful, clinically proven diagnostic and innovative digital solutions designed to help standardize care and protect all women from a preventable disease. The Roche Cervical Cancer Portfolio provides the right information at the right time with powerful clinical insights at each step with innovative solutions for screening, triage, and diagnosis.
The key to eliminating cervical cancer is more than a product. It’s a path.
Featured products
Benefits of Roche diagnostic solutions for managing cervical cancer
A leader in cervical cancer diagnosis, with trusted experience
A focus on the science of HPV has led to an evolution in cervical cancer screening strategies to better stratify patient risk, and improve outcomes. With the best scientists, and continuous commitment to the elimination of cervical cancer, we have built our organization around always striving to meet our customers’ needs and improve patient care.
Our holistic solution for cervical cancer screening, triage, diagnosis, and patient management is the most broadly recommended in guidelines to answer four questions that change lives and communities:
- Are they at risk?
- What do they need now?
- Am I certain of their diagnosis?
- How can I track and manage their care?
Roche's portfolio, now offering options for self-collection, reaches across geographies and cultures, so no one ever has to die from a preventable disease.
Roche has a long history as a scientific leader and innovator, driving cervical screening strategies and a change from the status quo, because we believe women deserve better. Roche continues to make significant investment in the cervical screening portfolio and in new product development, backed by strong clinical study evidence. In cervical cancer screening, with the goal of elimination in mind, we also recognize that digital innovations can bring efficiencies and scale to population-based programs, and with attention that also zeroes in at the patient level, we help to ensure that no one is left behind.
We launched the very first:
- FDA approved, CE mark, and WHO Prequalified cobas® HPV test for primary cervical screening
- FDA approved, CE mark dual-stain immunocytologic CINtec® PLUS Cytology triage test
- FDA cleared, CE mark diagnostic p16 biomarker immunohistochemistry test (CINtec® Histology)
- navify® Cervical Screening - Digital program management solution for cervical screening
Roche remains a leader in in vitro diagnostics (IVD), trusted by customers around the globe, with over 29 billion tests completed in 2022.4
Reaching more patients with a uniquely solutions-oriented, collaborative approach
Emphasizing Roche’s position as a collaborative partner, we work closely with labs to deliver high-impact solutions that meet their needs, their clinician customers’ needs, and corresponding population screening program requirements for patient database management. Our portfolio options are flexible to meet a guidelines-based patient care approach, with shared access across multiple site site locations, serving key stakeholders within the cervical screening ecosystem, including payers or policy makers.
With our ability for technical and healthcare consultancy, our support ranges from finding an optimal configuration to improving operational efficiency. For example, our navify® Cervical Screening tool is customizable to support local, country specific guidelines-based care from sample collection to diagnosis and treatment. Centralized data and analytics come with user-friendly dashboards that give at-your-fingertips recommendations for next step decisions using task-based lists. This helps clinicians save time, so that they can devote more energy to personalized patient care, and helps program managers better optimize population-based outcomes.
We leverage our partnerships with the Global Access Program and GoFurther to ensure that no matter where a woman lives, she has access to cervical cancer screening and treatment. Standardized patient and population care is within reach.
Strong medical value across the continuum of care
For cervical cancer screening, we're paving the way for a new standard of care based on our understanding of HPV’s role in disease prevention and progression. Our cutting-edge automation and integration streamline workflows, increase throughput, and accelerate turnaround times that ensure rapid and accurate results so patients know now if they are at increased risk.5
Our triage options for women with a positive high-risk HPV result further improve disease management decisions. Dual stain, biomarker technology, an immunocytologic triage test, can be performed using the same sample collected for HPV primary screening, meaning no additional patient exam is needed, and there is less potential loss to follow-up.
Finding and treating disease early helps drive better outcomes. Knowing whether to take clinical action immediately, or allow a woman’s body more time to clear the virus that causes cervical disease can be reassuring to clinicians and their patients.
Our platform options provide the flexibility to scale throughput and testing capabilities to increase value and access to new market opportunities, without requiring extensive training or subjective, technical expertise. This frees up skilled staff to dedicate more time to high-value tasks and collaborate with clinicians to improve patient care. Our digital innovations remove the burden of data entry, which minimizes the risk of human error or paper-based analytics and program population study reviews.
Our comprehensive menu of assays and solutions spans nearly the entire spectrum of routine testing, to better stratify risk for disease – along the continuum of screening, triage, diagnosis, and digital program or patient management.

HPV self-collection: Empowering women to protect themselves against cervical cancer
From the Peruvian Amazon to Ceredigion, Wales, learn how the power of innovation and collaboration can support efforts to eliminate cervical cancer.
Related health topics
References:
- World Health Organization. Cervical cancer fact sheet [Internet; cited 2024 July 3]. Available from: https://www.who.int/news-room/fact-sheets/detail/cervical-cancer.
- World Health Organization. WHO guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention [Internet; cited 2024 July 3]. Available from: https://www.who.int/publications/i/item/9789240030824.
- World Health Organization. WHO report on cancer: setting priorities, investing wisely and providing care for all [Internet; cited 2024 July 3]. Available from: https://www.who.int/publications/i/item/9789240001299.
- F. Hoffmann-La Roche Ltd. Annual Report 2022 [Internet; cited 2024 July 3]. Available from: https://assets.cwp.roche.com/f/126832/x/7cd4e2ba4c/ar22e.pdf.
- F. Hoffmann-La Roche Ltd. cobas HPV Qualitative nucleic acid test for use on the cobas 5800/6800/8800 Systems Method Sheet. (v2.0). 2024.