For localized information and support, would you like to switch to your country-specific website for {0}?
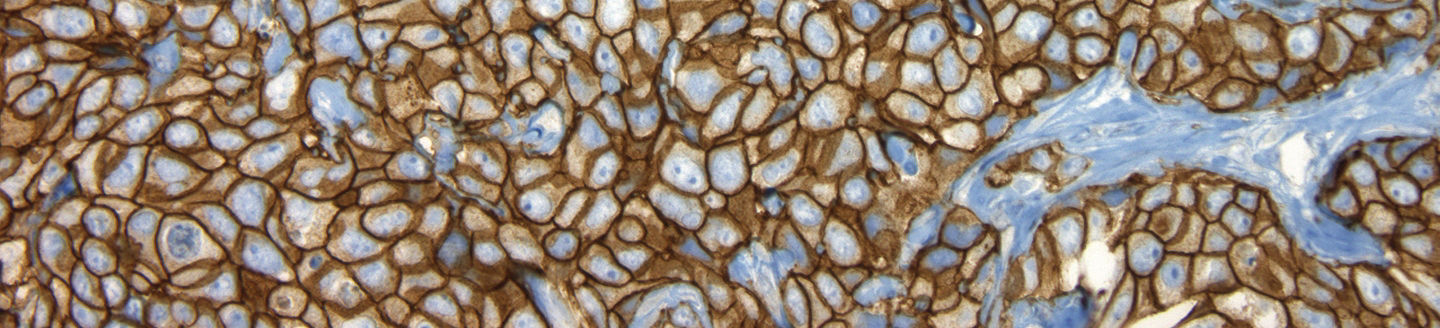
PATHWAY® anti-HER-2/neu (4B5) Rabbit Monoclonal Primary Antibody

Use left and right arrow keys to scroll between the tabs
Leading the way in HER2 testing
The use of pre-diluted PATHWAY anti-HER2/neu (4B5) Rabbit Monoclonal Primary Antibody1 (Roche HER2 (4B5)), in combination with the fully automated BenchMark IHC/ISH slide staining instrument, standardizes all IHC processes from baking through staining, and reduces the possibility of human error.1 It also minimizes inherent variability resulting from individual reagent dilution and other processes found in manual and semi-automated IHC methods.
The Roche HER2 (4B5) clone* empowers you to:
- Achieve consistently high proficiency assessment scores with the Roche HER2 (4B5) clone, compared to other clones2
- Employ the most widely adopted and reliable HER2 IHC primary antibody2
- Demonstrate high concordance with HER2 FISH3,4
Consistent performance and superior quality
The Roche HER2 (4B5) clone* has shown the most consistent performance and superior quality when compared to other on-market HER2 clones.**
*Refers to the PATHWAY anti-HER2/neu (4B5) Rabbit Monoclonal Primary Antibody and VENTANA® anti-HER2/neu (4B5) Rabbit Monoclonal Primary Antibody products.
**Based on data from a leading external quality assessment scheme.2

References
- PATHWAY anti-HER2/neu (4B5) Rabbit Monoclonal Primary Antibody Package Insert (14427US Rev K), 2025.
- NordiQC Assessments. https://www.nordiqc.org/epitope.php?id=11. Accessed January 28, 2025.
- Mayr D, et al. Comprehensive immunohistochemical analysis of Her-2/neu oncoprotein overexpression in breast cancer: HercepTest™ (Dako) for manual testing and Her2/neuTest 4B5 (VENTANA) for VENTANA BenchMark automatic staining system with correlation to results of BenchMark automatic staining system with correlation to results of fluorescence in situ hybridization (FISH). Virchows Archiv. 2009; 454(3):241–248.
- Brügmann A, Lelkaitis G, Nielsen S, et al. Testing HER2 in breast cancer: a comparative study on BRISH, FISH, and IHC. Appl Immunohistochem Mol Morphol. 2011;19(3):203-211.
- PATHWAY anti-HER2/neu (4B5) Rabbit Monoclonal Primary Antibody Package Insert (14427EN Rev H), 2022.