For localized information and support, would you like to switch to your country-specific website for {0}?
VENTANA® anti-HER2/neu (4B5) Rabbit Monoclonal Primary Antibody

Use left and right arrow keys to scroll between the tabs
Leading the way in HER2 testing
The use of pre-diluted VENTANA anti-HER2/neu (4B5) Rabbit Monoclonal Primary Antibody1, in combination with the fully automated BenchMark IHC/ISH slide staining instrument, standardises all IHC processes from baking through staining, and reduces the possibility of human error. It also minimizes inherent variability resulting from individual reagent dilution and other processes found in manual and semi-automated IHC methods.
The Roche HER2 (4B5) clone* empowers you to:
- Achieve consistently high proficiency assessment scores compared to other clones2
- Employ the most widely adopted and reliable HER2-IHC primary antibody2
- Demonstrate high concordance with HER2 FISH3,4

VENTANA® HER2 (4B5) Rabbit Monoclonal Primary Antibody RxDx
CE-IVD
Building on the same proven technology of the widely adopted HER2 (4B5) assay, Roche has delivered the only test approved to identify HER2-low breast cancer patients - helping match even more women with highly effective personalised therapies.
Consistent performance and superior quality
The Roche HER2 (4B5) clone* has shown the most consistent performance and superior quality when compared to other on-market HER2 clones.2
*Refers to the PATHWAY anti-HER2/neu (4B5) Rabbit Monoclonal Primary Antibody and VENTANA anti-HER2/neu (4B5) Rabbit Monoclonal Primary Antibody products.

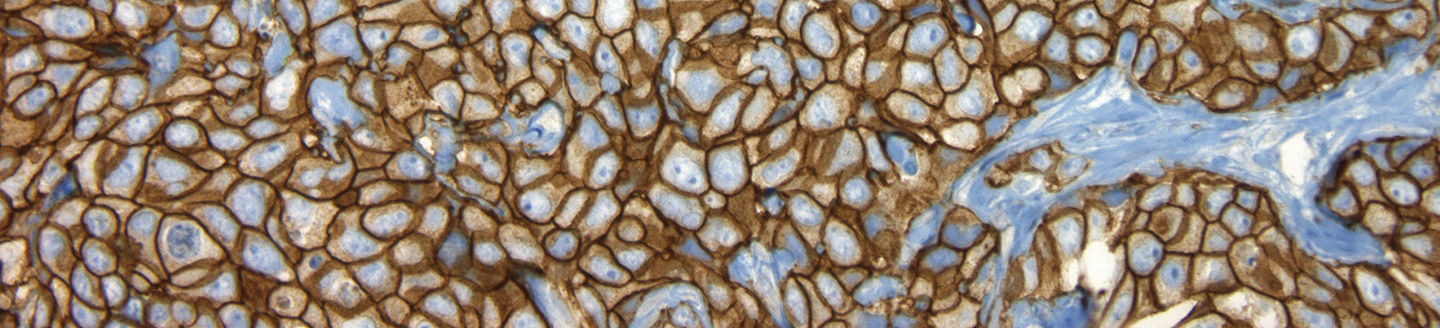
Criteria for intensity and pattern of cell membrane staining with VENTANA anti-HER2/neu (4B5) Rabbit Monoclonal Primary Antibody in breast carcinoma:
View Full TableCriteria for intensity and pattern of cell membrane staining with VENTANA anti-HER2/neu (4B5) Rabbit Monoclonal Primary Antibody in breast carcinoma:
| Staining Pattern | Score (Report to Treating Physician) |
HER2 Staining Assessment |
|---|---|---|
| No membrane staining is observed | 0 | Negative |
| Faint, partial staining of the membrane in any proportion of the cancer cells | 1+ | Negative |
| Weak complete staining of the membrane, > 10% of cancer cells | 2+ | Equivocal* |
| Intense complete staining of the membrane, > 10% of cancer cells | 3+ | Positive |
Criteria for intensity and pattern of cell membrane staining with VENTANA anti-HER2/neu (4B5) Rabbit Monoclonal Primary Antibody in gastric carcinoma:
View Full TableCriteria for intensity and pattern of cell membrane staining with VENTANA anti-HER2/neu (4B5) Rabbit Monoclonal Primary Antibody in gastric carcinoma:
Staining Pattern - Resection Specimen |
Score (Report to requesting physician) | HER2 Staining Assessment |
|---|---|---|
| No reactivity or membranous reactivity in < 10% of tumour cells | 0 | Negative |
| Faint/barely perceptible membranous reactivity in ≥ 10% of tumour cells; cells are reactive only in part of their membrane | 1+ | Negative |
| Weak to moderate complete, basolateral or lateral membranous reactivity in ≥ 10% of tumour cells | 2+ | Equivocal** |
| Strong complete, basolateral or lateral membranous reactivity in ≥ 10% of tumour cells | 3+ | Positive |
References
- PATHWAY/VENTANA anti-HER2/neu (4B5) Rabbit Monoclonal Primary Antibody Package Inserts, 2019/2022.
- NordiQC Assessments. https://www.nordiqc.org/epitope.php; accessed November 2023
- Mayr D, et al. Comprehensive immunohistochemical analysis of Her-2/neu oncoprotein overexpression in breast cancer: HercepTest™ (Dako) for manual testing and Her-2/neuTest 4B5 (VENTANA) for VENTANA BenchMark automatic staining system with correlation to results of BenchMark automatic staining system with correlation to results of fluorescence in situ hybridization (FISH). Virchows Archiv. 2009; 454(3):241–248.
- Brügmann A, Lelkaitis G, Nielsen S, et al. Testing HER2 in breast cancer: a comparative study on BRISH, FISH, and IHC. Appl Immunohistochem Mol Morphol. 2011;19(3):203-211.